What you need to know about antimicrobial efficacy test standards for hard surfaces
As the understanding of antimicrobial materials has improved, regulators and working groups such as the US Environmental Protection Agency (EPA) and the Organisation for Economic Co-operation and Development (OECD) have recognised that there are no standardized methods for the determination of efficacy under conditions typical of the environment under which a material is expected to be employed. This is especially the case with regard to indoor surfaces and touch points.
The OECD Working Group under the Inter-Organisation Programme for the Sound Management of Chemicals (IOMC) represents the interests of a number of countries and regions. It has proposed a tiered system approach1 with Tier One test methods providing proof of principle and Tier Two methods more closely representing in-use environmental conditions.
Tier One testing
Tier One tests are adequately covered by standards such as BS EN ISO 22196: Measurement of antibacterial activity on plastics and other non-porous surfaces. This establishes the basic effectiveness of a material under warm, damp conditions: 35oC and >90% humidity over a period of 24 hours. This, and the Japanese Industrial Standard on which it is predicated (JIS Z 2801), is the basis on which most early technical and marketing claims were made and often persist in promotional literature. This is now seen as inappropriate, or possibly dangerous, in that efficacy under the test conditions has been shown2 not to persist under typical indoor settings where humidity is often 50% and temperatures only 22 – 25oC.
Tier Two tests are still in the committee stage of various Standards Organisations and the OECD recommends a pragmatic, best practice approach to test protocols while new standards are under development. OECD also recommends allowing the inoculum to dry and significantly reduced test times. The development of appropriate Tier Two test standards will enable better comparisons and the wider implementation of these technologies.
Antimicrobial Copper testing
Through dialogue with the EPA, a set of three Tier Two methods was established based upon existing tests for liquid disinfectants designed to be applied to hard surfaces. In particular, pre-drying of inocula on test coupons and a 2 hour assessment period were recommended. Its development included extensive discussions with experts in the infection control community, the Association for Professionals in Infection Control and Epidemiology (APIC) and the American Society for Healthcare Environmental Services (ASHES). These test methods incorporated the more recent OECD recommendations regarding test conditions and times. See EPA Tests and Approval for details of the three EPA tests.
The table below compares the conditions of the Tier Two 'Efficacy as a Sanitizer' EPA test with the Tier One JIS Z 2801 (ISO 22196)
|
Efficacy test conditions |
Antimicrobial Copper |
Silver-containing coatings |
|---|---|---|
| Protocol | EPA test protocol | Japanese Industrial Standard
JIS Z 2801 (ISO 22196) |
| Time | 2 hours | 24 hours |
| Exposure | Open to atmosphere | Plastic film to retain moisture |
| Drying time | 10 - 20 mins | Remains wet |
| Temperature | Room temperature
(~20°C / 68°F) |
Close to body temperature
(~35°C / 95°F) |
| Relative humidity | Ambient (~50%) | >90% |
| Inoculum level | High (>10 million CFU) | Lower (500,000 – 1 mi CFU) |
Table comparing conditions of EPA Efficacy as a Self-Sanitizer test with JIS Z 2801 (ISO 22196)
Industry best practice antimicrobial efficacy test
The EPA Efficacy as a Self-Sanitizer Tier Two (2-hour kill) test has been developed into a detailed protocol by AlControl Laboratories to make it accessible to all those looking for an appropriate test for hard surfaces in the absence of a published standard. See the Find Services page for labs familiar with this test protocol.
The protocol is provided in the downloads below along with the certificates of analysis for 13 commonly available approved copper alloys. The test certificates include the alloy ISO designation, test results (including controls) and a statement confirming the test has been passed. The alloys are all those listed in Publication 214: Antimicrobial Copper Alloys – Guidance on Selection.
Further test development
In order to better understand the broad spectrum efficacy and mechanisms at play, developments of the Tier Two tests have been used.
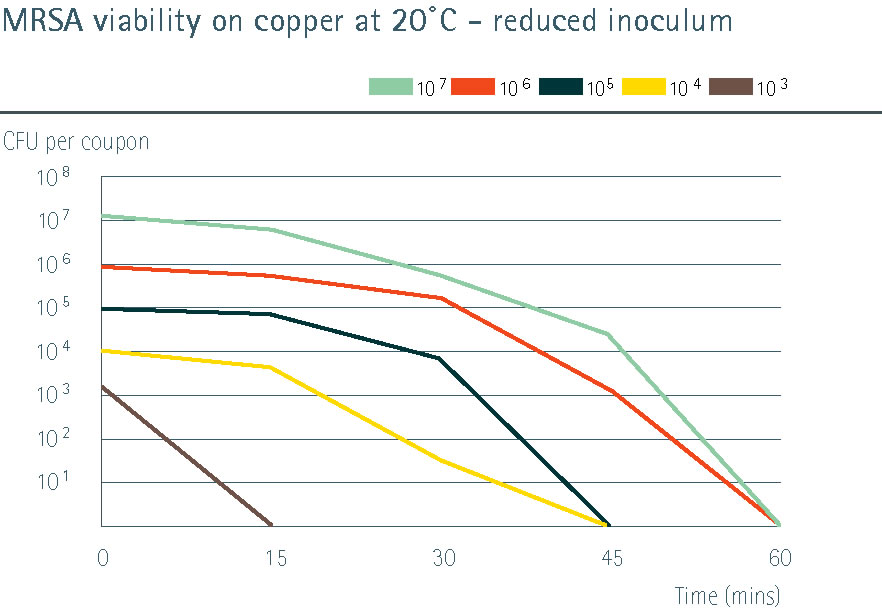
Instead of using the very high microbe count in the inoculum (as originally specified in the liquid disinfectant test), progressively reduced numbers of MRSA bacteria were put onto a set of test samples3. This showed that when more real-world contamination levels were used (103 CFU/cm2), copper alloys were able to kill the microbes in 15 minutes rather than the 2 hour test period required by the EPA methods.
Click to enlarge
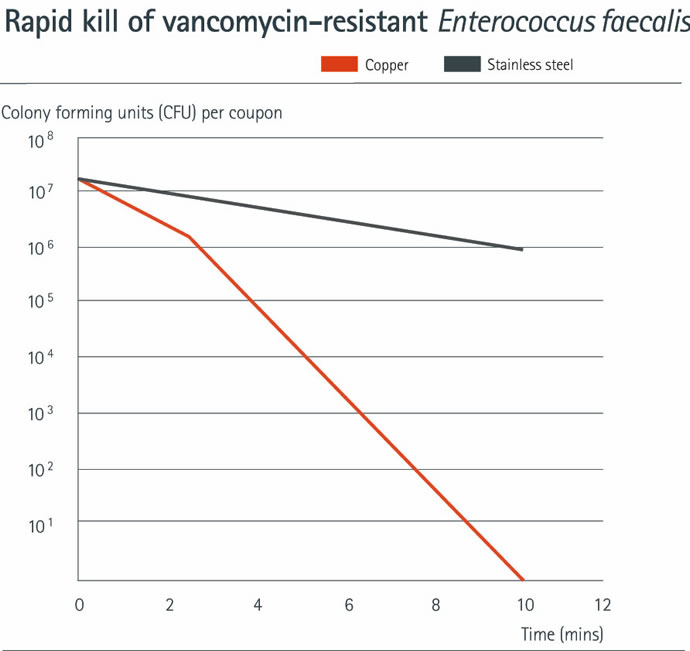
At the University of Southampton, Environmental Health Unit, work has shown that by reducing the inoculum volume from 20 µl to just 2 µl the drying phase was reduced to a minimum whilst still maintaining excellent repeatability. This small droplet size closely approximates to a human touch contamination event. Perhaps surprisingly, this showed that copper alloys worked significantly faster (10 million bacteria killed in less than 10 minutes) under these drier deposition conditions than the EPA method4. Logically, using a real-world microbe inoculum, kill times would be even faster.
Click to enlarge
Further test development
Standards bodies and regulators are working on developing new standards based on test methods that reflect in use conditions.
Copper and copper alloys are engineering materials that are durable, colourful and recyclable and are widely available in various product forms suitable for a range of manufacturing purposes. Copper and its alloys offer a suite of materials for designers of functional, sustainable and cost-effective products.
Copper and certain copper alloys have intrinsic antimicrobial properties (so-called ‘Antimicrobial Copper’) and products made from these materials have an additional, secondary benefit of contributing to hygienic design. Products made from Antimicrobial Copper are a supplement to, not a substitute for standard infection control practices. It is essential that current hygiene practices are continued, including those related to the cleaning and disinfection of environmental surfaces.
References
- OECD Guidance Document on Quantitative Methods for Evaluating the Activity of Microbicides used on Hard Non-Porous Surfaces. ENV.JM.MONO(2014)18.
- Effects of Temperature and Humidity on the Efficacy of Methicillin-resistant Staphylococcus Aureus Challenged Antimicrobial Materials Containing Silver and Copper. H T Michels, J O Noyce, and C W Keevil. Letters in Applied Microbiology, 49 (2009) 191-19.
- Potential use of copper surfaces to reduce survival of epidemic methicillin-resistant Staphylococcus aureus in the healthcare environment. J O Noyce, H Michels and C W Keevil. Journal of Hospital Infection, Vol 63, Issue 3, pp 289-297, July 2006.
- Mechanism of Copper Surface Toxicity in Vancomycin-Resistant Enterococci following Wet or Dry Surface Contact. S L Warnes and C W Keevil. Applied and Environmental Microbiology, September 2011.