Antimicrobial Copper is the only class of solid metal touch surfaces registered by the US Environmental Protection Agency (EPA) to continuously kill bacteria* that cause infections and pose a risk to human health.
EPA Tests
Through dialogue with the EPA a set of three test methods was established based upon existing tests for liquid disinfectants designed to be applied to hard surfaces. It’s development included extensive discussions with experts in the infection control community, the Association for Professionals in Infection Control and Epidemiology (APIC), the American Society for Healthcare Environmental Services (ASHES).
Copper alloys are a new concept in environmental disinfection and act continuously throughout the lifetime of the material when cleaned regularly and as a supplement to routine cleaning and disinfection programmes. See the section on Efficacy Tests and Standards for further information.
The three EPA approved Good Laboratory Practice (GLP) test protocols used to register Antimicrobial Copper with public health claims are:
- Efficacy as a Sanitizer - which measures viable bacterial count under typical indoor conditions after two hours. This method has been adopted as industry best practice and a downloadable version can be found on the Efficacy Tests and Standards page.
- Residual Self-Sanitising Activity - which measures bacterial count before and after six wet and dry wear cycles during which bacteria are added in a standard wear apparatus (shown as a schematic in Figure 1).
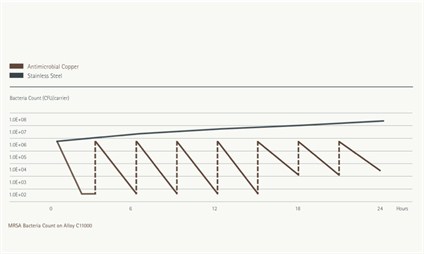
- Continuous Reduction of Bacterial Contaminants - which measures bacteria after inoculating an alloy surface eight times in a 24-hour period without intermediate cleaning or wiping.
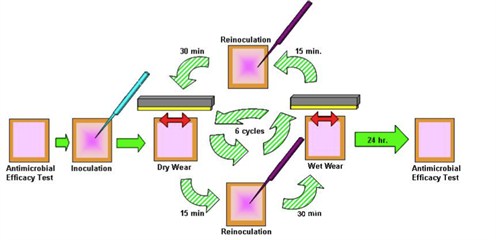
Figure 1: Residual Self-Sanitizing test protocol schematic.
Figure 2: Continuous Reduction test results for MRSA on Antimicrobial Copper C11000 and Stainless Steel S30400. Each inoculation adds 650,000 CFUs.
Click on graph to enlarge
The results of the 216 GLP tests, involving three test protocols, two to three lots of six different alloys, and six bacteria*, are summarised in Table 1. In both the Efficacy as a Sanitizer test and Residual Self-
Sanitizing test (wear test), a reduction in live bacteria greater than 99.9% is seen in all seventy two tests when compared to stainless steel (S304). In the Continuous Reduction of Bacterial Contaminants test, a reduction of greater than 99.9% is found in sixty-three out of the seventy-two tests, again when compared to S304. In the remaining nine tests, reductions ranged from 99.3% to 99.9%.
In summary, a reduction greater than 99.9% was seen on 207 out of 216 tests. The reduction seen in the remaining nine tests ranged from 99.3% to 99.9%. These results indicate that the antimicrobial response of copper alloys is effective, enduring and reproducible.
|
|
Group |
Alloy |
%Cu |
S.aureus |
E.aerogenes |
MRSA |
P.aeruginosa |
E. coli O157:H7 |
|
Efficacy as a sanitiser |
I |
C110 |
99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
II |
C510 |
94.8 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
III |
C706 |
88.6 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
IV |
C260 |
70 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
V |
C752 |
65 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
VI |
C280 |
60 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
|
|
|
|
|
|
|
|
|
Residual Self Sanitising |
I |
C110 |
99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
II |
C510 |
94.8 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
III |
C706 |
88.6 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
IV |
C260 |
70 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
V |
C752 |
65 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
VI |
C280 |
60 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
|
|
|
|
|
|
|
|
|
Continuous Reduction |
I |
C110 |
99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
II |
C510 |
94.8 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
III |
C706 |
88.6 |
>99.9 |
>99.9 |
99.9 |
>99.9 |
>99.9 |
|
|
IV |
C260 |
70 |
99.6 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
V |
C752 |
65 |
99.7 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
VI |
C280 |
60 |
99.8 |
>99.9 |
99.9 |
>99.9 |
>99.9 |
Table 1: Average Percent Reduction of Bacterial Contamination (Good Laboratory Practice Studies)
After many years of independent laboratory testing, followed by the additional rigorous testing under EPA approved protocols, 275 copper alloys (including brasses and bronzes) were registered as public health antimicrobial products by EPA on February 29, 2008. The list has since broadened, now including more than 500 registered alloys.
Public health products are intended to control microorganisms associated with infections or other adverse effects in humans.
Silver-containing coatings do not have public health registration from EPA. These products are marketed under a "Treated Article Exemption" which means the antimicrobial additive only may protect the product itself from degradation and odour caused by non-specific organisms.
Silver-containing coatings do not continuously kill bacteria that cause infections. Antimicrobial Copper surfaces do.
EPA Registration
Antimicrobial Copper alloy products can claim to kill 99.9% of disease causing bacteria* within two hours, when cleaned regularly and as a supplement to routine cleaning and disinfection programmes.
EPA registration is a legal US Federal Government decision acknowledging the efficacy of Antimicrobial Copper products against the 6 following disease-causing bacteria:
- E. coli O157:H7, a food-borne pathogen that has been associated with large-scale food recalls;
- Methicillin-Resistant Staphylococcus aureus (MRSA), one of the most virulent strains of antibiotic-resistant bacteria and a common culprit of hospital- and community-acquired infections;
- Staphylococcus aureus, the most common of all bacterial staphylococcus (i.e. staph) infections that can cause life-threatening diseases, including pneumonia and meningitis;
- Vancomycin-Resistant Enterococcus faecalis (VRE), an antibiotic resistant organism responsible for 4% of all Healthcare-Associated Infection;
- Enterobacter aerogenes, a pathogenic bacterium commonly found in hospitals that causes opportunistic skin infections and impacts other body tissues; and,
- Pseudomonas aeruginosa, a bacterium that infects the pulmonary tracts, urinary tracts, blood, and skin of immunocompromised individuals.
In the US, Antimicrobial Copper products can only be sold by registered manufacturers using registered copper alloys.
To view the registered public health claims, visit the US section of this website Public Health Claims.
International Research
Research on the antimicrobial efficacy of copper alloys has been carried out and verified at leading institutions around the world, including the UK, US, Chile, Greece, Germany, Japan and South Africa. Results have been peer reviewed and published in a number of papers and reviews. See the Research Groups and Scientific References pages.
Copper and copper alloys are engineering materials that are durable, colourful and recyclable and are widely available in various product forms suitable for a range of manufacturing purposes. Copper and its alloys offer a suite of materials for designers of functional, sustainable and cost-effective products.
Copper and certain copper alloys have intrinsic antimicrobial properties (so-called ‘Antimicrobial Copper’) and products made from these materials have an additional, secondary benefit of contributing to hygienic design. Products made from Antimicrobial Copper are a supplement to, not a substitute for standard infection control practices. It is essential that current hygiene practices are continued, including those related to the cleaning and disinfection of environmental surfaces.
*Antimicrobial Copper is the only solid metal surface material to have efficacy data independently verified through the US Environmental Protection Agency (EPA) registration which supports the claim to continuously kill more than 99.9% of the bacteria that cause HCAIs within two hours of contact. Organisms tested are MRSA, Staphylococcus aureus, Enterobacter aerogenes, Pseudomonas aeruginosa, E. coli O157:H7 and Vancomycin-resistant Enterococcus faecalis.
Reference
- Antimicrobial Regulatory Efficacy Testing of Solid Copper Alloy Surfaces in the USA. H T Michels and D G Anderson, pp 185-190, Metal Ions in Biology and Medicine: Vol 10, Eds Ph Collery, I Maymard, T Theophanides, L Khassanova, T Collery. John Libbey Eurotext, Paris © 2008.